Preoperative inflammation markers and IDH mutation status predict glioblastoma patient survival
2018-10-23 11:49 作者:三博腦科醫(yī)院

作者:王鵬斐
ABSTRACT
Recent studies suggest that inflammation response biomarkers are prognostic indicators of solid tumor outcomes. Here, we quantify the prognostic value of the neutrophil-to-lymphocyte ratio (NLR),platelet-to-lymphocyte ratio (PLR), and lymphocyte-to-monocyte ratio (LMR) in glioblastomas (GBMs),taking into consideration the role of the isocitrate dehydrogenase (IDH) mutation status. We examined 141 primary glioblastomas (pGBMs) and 25 secondary glioblastomas (sGBMs). NLRs, PLRs, and LMRs were calculated before surgery. IDH mutations were detected immunohistochemically after tumor resection, and patients’ clinical outcomes were analyzed after classification into GBM, pGBM, and IDH-wild type glioblastoma (IDH-wt GBM) groups. To make comparisons, we set cutoffs for NLR, PLR and LMR of 4.0,175.0, and 3.7, respectively. In a multivariate analysis, both NLR (HR=1.712, 95% CI 1.026-2.858, p=0.040) and PLR (HR=2.051, 95% CI 1.288-3.267, p=0.002) had independent prognostic value. While a low NLR was associated with a better prognosis only in the IDH-wt GBM group, PLR was predictive of patient survival in the GBM, pGBM, and IDH-wt GBM groups. By contrast, LMR exhibited no prognostic value for any of the 3 types of GBM.
INTRODUCTION
Glioblastomas are the most common brain malignancies, accounting for 15.1% of the total central nervous system tumors.Glioblastmas are classified as either primary glioblastomas (pGBMs) or secondary glioblastomas (sGBMs), which develop from lower-grade gliomas. The discovery that isocitrate dehydrogenase (IDH) mutations are more common in sGBMs was one of the most significant advancements in the understanding of gliomas [3, 4]. Patients with glioblastomas carrying IDH mutations or wildtype IDH, exhibited large differences in prognosis, age, and genetic alternations [5-9].
Mounting evidence suggests an important role for inflammation in tumor development [10, 11]. The development of gliomas, in particular, is closely associated with inflammation status and immune response [12, 13]. NLRs, PLRs, and LMRs are markers of host inflammation. A high NLR and PLR and low LMR are closely associated with a poor prognosis in solid malignancies, including gastrointestinal tumors, prostate cancer, and lung cancer [14-19]. While a low preoperative NLR closely correlates with lower glioma grade and better clinical outcome [20-22], there are no published data assessing the role of PLR or LMR in gliomas. Additionally, the role of the NLR in gliomas requires further study, due to the limited
number of cases in previous studies [21, 22]. We therefore hypothesized that the inflammation status would likely vary according to the IDH mutation status, and could serve as a prognostic indicator. Herein, we investigated the prognostic value of NLRs, PLRs, and LMRs, in both pGBMs and sGBMs. The characteristics of NLRs,PLRs, and LMRs are also described here, taking into consideration IDH mutation status.
RESULTS
Patient characteristics
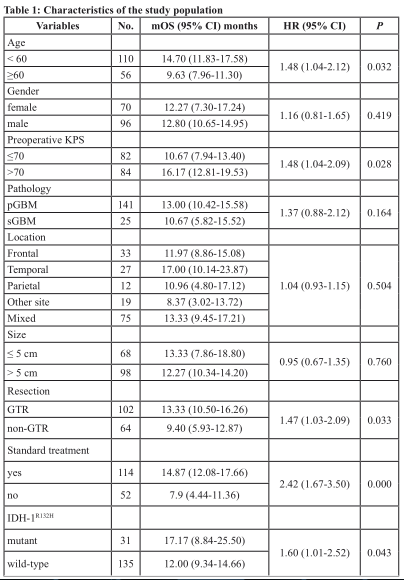
We enrolled 166 patients with GBMs in the present study, including 70 females and 96 males. The age of the patients ranged from 18 to 80 years with an average of 52.1 ± 0.984 years. The frequency of IDH mutations was9.9% (14/141) among pGBMs and 68% (17/25) among sGBM. The Karnofsky score (KPS), tumor location,surgical resection, and molecular markers are described in Table 1.
The median overall survival (OS) did not differ with respect to gender, tumor location, or tumor size (Table 1). Patients carrying IDH-1 R132H mutations had better prognoses [median 17.17 months (95% CI 8.84 – 25.50) vs. 12.00 months (95% CI 9.34 – 14.66); p = 0.041].Higher preoperative KPS, surgical resection, and full treatment with radiochemotherapy were also associated with better clinical outcomes (Table 1). Among patients meeting our inclusion criteria, these clinical characteristics varied within a reasonable range in previous reports [1, 2,5, 8].
No association between NLR, PLR, or LMR andIDH mutations
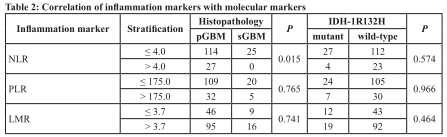
We observed that NLR was elevated more frequently in pGBMs than sGBMs (p = 0.015). However, PLR did not differ between pGBMs and sGBMs (p=0.765), nor did LMR (p = 0.741, Table 2). No difference was found in NLR (p = 0.574), PLR (p = 0.966) or LMR (p =0.564) with respect to IDH mutation status. We found no significant correlation between NLR or PLR and patients’ age, gender, KPS, tumor location or size, or molecular markers. (Data not shown)
Analysis of NLR, PLR, and LMR in predicting outcomes
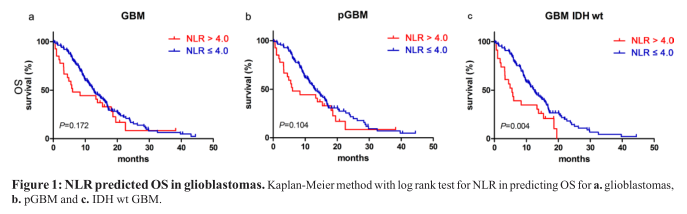
We found that NLR had no significant prognostic value for patients with glioblastomas [12.80 months (95% CI 10.40–15.20) vs. 6.03 months (95% CI 1.16–10.90);p=0.172, Figure 1a] and those in the pGBM group [13.30 months (95% CI 1.91–15.69) vs. 6.03 months (95% CI 1.16–10.90); p=0.104, Figure 1b]. However, patients who had a NLR ≤ 4.0 and were in the group carrying IDH-1 R132H-wt had better prognoses [12.60 months (95% CI 10.22–14.98) vs. 5.50 months (95% CI 3.40–7.60); p=0.004, Figure 1c].
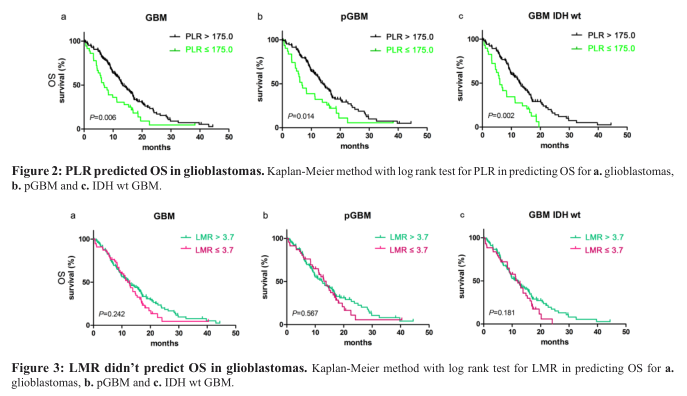
The median OS of 13.33 months (95% CI 11.25–15.41) for patients with PLR ≤ 175.0 was longer than the 7.00 months (95% CI 4.22–9.78) for patients with PLR > 175.0 (p=0.006, Figure 2a). PLR ≤ 175.0 was also associated with better clinical outcome in the pGBM group [14.27 months (95% CI 11.83–16.71) vs. 6.80 months (95% CI 3.57–10.03); p=0.014, Figure 2b] and the IDH-1 R132H-wt group [13.00 months (95% CI 10.84–15.16) vs. 6.03 months (95% CI 3.38–7.68); p=0.002, Figure 2c].
The median OS did not differ significantly between groups stratified based on LMR ≥ 3.7 [12.00 months (95% CI 9.94–14.06) vs. 12.60 months (95% CI 9.19–16.00); p=0.242, Figure 3a]. No significant prognostic value for LMR ≥ 3.7 was observed in patients with pGBM [13.83 months (95% CI 10.74–16.92) vs. 12.60 months (95% CI 8.82–16.39); p=0.567, Figure 3b], nor with IDH-1 R132H-wt [12.00 months (95% CI 9.56–14.44) vs. 12.27 months(95% CI 9.07–15.47); p=0.181, Figure 3c].
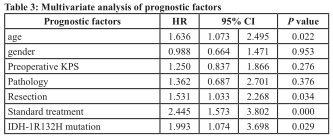
Multivariate analysis indicated that age (p=0.022),extent of resection (p=0.034), full treatment (p=0.000) and IDH mutations (p=0.029) were independent prognostic factors after taking age, gender, KPS, extent of resection, full treatment, pathology and IDH mutations into account (Table 3). However, NLR, PLR, and LMR were strongly correlated with each other [(Spearman’s rho coefficients of 0.631 (NLR vs PLR, p=0.000), -0.344 (NLR vs LMR, p=0.000) and -0.240 (PLR vs LMR, p=0.002)]. All three factors were analyzed in the multivariate analysis adjusted by the above 7 factors. Both NLR (HR=1.714, 95% CI 1.026-2.864, p=0.039) and PLR (HR=2.068, 95% CI 1.296-3.300, p=0.002) were indicated to be independent prognostic factors. However, we found that LMR had no independent prognostic value for OS (HR=0.733, 95% CI 0.481-1.119, p=0.150).
DISCUSSION
In the present study, we first assessed the prognostic value of NLR, PLR, and LMR in glioblastomas, taking into account IDH mutation status. NLR and PLR were independent prognostic biomarkers for patient outcomes and therefore confirm published data from glioblastomas [21-23] and other malignancies [14, 17, 24]. However, LMR was not predictive of OS in glioblastomas. We found that reduced NLR was associated with improved OS in pGBM, though the significance was not as obvious as in previous studies [21-23]. This difference may be explained by differences among previous studies. While NLR was established as a prognostic marker for malignancies in some studies [14, 17], others failed to observe a significant prognostic value for NLR in breast cancer [25], gastric cancer [26], and prostate cancer [27]. It is likely that not all patients received thesame treatment in each study. In our study, all patients underwent surgery. Among them, 61.44% (102/166) had a gross treatment resection, and 68.67% (114/166) received radiochemotherapy according to Stupp’s protocol. In other studies, the proportion of patients choosing each treatment strategy, which included biopsy, surgery, and radiochemotherapy, varied [21-23]. Additionally, the inclusion of IDH mutations was superior to histopathology alone for classifying glioblastomas [28]. We concluded that IDH-wt glioblastomas had better defined clinical outcomes than pGBM. Our multifactorial analysis first took IDH mutations as prognostic indicators, and NLR remained an independent prognostic biomarker. Interestingly, we observed that higher NLRs were more frequent in pGBM than sGBM. Zadora et al. reported that NLR values differed among glioma grades and were highest in glioblastomas [20]. Secondary glioblastoma originates from a lower-grade glioma. This likely explains why NLRs were low in sGBM. Furthermore, we also found that elevated PLR correlated closely with poor prognosis in our study, which is consistent with Han’s results [23]. The prognostic value of PLR was found not only in IDH-wt glioblastomas, but also in glioblastomas and pGBMs in our study.
The mechanism underlying the prognostic role of NLR/PLR remains unclear in glioblastomas. The blood- brain-barrier is frequently disrupted in glioblastomas, allowing circulating lymphocytes to cross [29]. Moreover, NLR was significantly related to high neutrophil and low CD3 + T-cell infiltration into glioblastomas [23]. Tumor- infiltrating lymphocytes (TILs), which are predominately regulatory T cells in the glioblastoma microenvironment, could suppress immune responses [30]. However, recent studies indicate that TILs are not sufficient to mediate the glioblastoma-related immune suppression [31-33]. PD-L1 (programmed death ligand 1) and CTLA-4 (Cytotoxic T-lymphocyte-associated protein 4) have been identified as alternatives for immunosuppression in glioblastomas [34, 35]. Additionally, PD-L1 proteins were detected in the microenvironment of glioblastomas or brain metastases [36-38]. These results suggest a more complicated immunosuppressive mechanism in glioblastomas, which is likely to involve both systemic and local microenvironmental inflammation. We therefore propose that a complete score system is needed to fully assess systemic inflammation status, involving an immunosuppressive biomarker in the microenvironment.
MATERIALS AND METHODS
Study population
This retrospective study was conducted to investigate the relationship between NLR/PLR andglioblastomas. The inclusion criteria were: (1) Surgical treatment in Sanbo Brain Hospital from 2009 to 2014, (2) the presence of histologically confirmed supratentorial
glioblastomas, (3) operative blood test performed prior to corticosteroid treatment or no chemotherapy within the previous month, (4) available medical records indicating the patient’s age, gender, molecular pathology and follow-up data, and (5) provided informed consent before the investigation. Ultimately participating in the study were 166 patients, including 141 with pGBMs and 25 with sGBMs. The Stupp protocol was used for concurrent chemoradiotherapy followed by consolidation chemotherapy with temozolomide [39]. OS time was defined as the interval from surgery until death or the latest follow-up. All experiments using human tissues were approved by the Institutional Review Board of Sanbo Brain Hospital.
Immunohistochemistry
Immunohistochemistry was used for detection of IDH mutations. The procedures were performed as described previously [9] using primary antibodies against IDH1 R132H (Dianova 1:100) . The cutoff values were 10% for IDH-1 R132H mut .
Statistics
Data are presented as means ± SEM. SPSS 22.0 was used for all the other statistical analyses. The χ2 test was used to evaluate the correlations between NLR, PLR and LMR and the patients’ clinical characteristics. Survival curves were analyzed using the Kaplan-Meier method and the Breslow test. Values of p<0.05 (two-sided) were considered statistically significant.
ACKNOWLEDGMENTS
This work was supported by grants from National Key Technology Research and Development Program of the Ministry of Science and Technology of China (No.2014BAI04B01) and the National Youth Science Fund from China (No.81302200).
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.




 京公網(wǎng)安備 11010802035500號(hào)
京公網(wǎng)安備 11010802035500號(hào)