Prognostic and predictive value of p53 in low MGMT expressing glioblastoma treated with surgery, radiation and adjuvant temozolomide chemotherapy
2018-08-30 11:19 作者:三博腦科醫(yī)院
Shouwei Li1, Tao Jiang 2
1 Department of Neurosurgery, Beijing Sanbo Brain Hospital, Capital Medical University, Beijing, China
2 Glioma Treatment Center, Beijing Tiantan Hospital, Affiliated to Capital University of Medical Sciences, Beijing 100050, China
Correspondence and reprint requests to:
Tao Jiang M.D., Ph.D.
Glioma Treatment Center, Beijing Tiantan Hospital, Affiliated to Capital University of Medical Sciences, Beijing 100050, China
Email: taojiang1964@yahoo.com.cn
Tel:+86-10-83135647
Fax: +86-10-83135647
Abstract
Objective: To assess the prognostic and predictive significance of p53 protein expression in low O6-methylguanine-DNA methyltransferase (MGMT) expressing glioblastomas (GBM) treated with combined therapy.
Methods: The authors reviewed the clinical outcomes of 46 low MGMT expressing GBM patients who had undergone surgery, conventional local radiotherapy and TMZ chemotherapy. Correlation between p53 expression level and clinical outcomes were analyzed. with univariate and multivariate cox model.
Results: Patients with low p53 expression had a significantly improved progression free survival (PFS) (P = 0.015) and overall survival (OS) (P = 0.047) compared to those with high expression. On both univariate and multivariate analysis, low p53 expression persisted as significant independent favorable prognostic factor for PFS (P = 0.017). Preoperative KPS score (P = 0.029), tumor resection extent (P = 0.045) and p53 expression level (P = 0.038) were significant independent prognostic factors for OS.
Conclusion: In these low MGMT expressing GBM patients with combined treatment, low p53 expression was a significant independent favorable prognostic factor for both PFS and OS. In addition to MGMT, p53 may be another stratification variable in the future therapeutic trials.
Keywords: Glioblastoma Multiform; Temozolomide; P53; Prognosis
INTRODUCTION
Glioblastoma Multiform (GBM) is the most frequent primary malignant brain tumor in adults. Its prognosis is particularly disappointing with a median life expectancy less than a year even treated with the most aggressive regimens 1. Postoperative radiotherapy (RT) has been recognized as standard therapy for long time based on six randomized studies published between 1976 and 1991 2. The role of alkylating agent based chemotherapy has been controversial 3-8 until the appearance of EORTC trial 26981, which demonstrated unequivocally that addition of temozolomide (TMZ) to RT provided a statistically significant and clinically meaningful survival benefit for GBM patients, producing an increase in the median survival time from 12.1 to 14.6 months and in the two-year survival rate from 10 to 26% 9. The results were further confirmed when the patients were classified according to recursive partitioning analysis class 10. While postoperative radiotherapy in combination with TMZ chemotherapy has become the first choice for newly diagnosed GBM patients, chemotherapy resistance is still intractable 11-13.
DNA repair protein O6-methylguanine-DNA methyltransferase (MGMT) is an important mechanism involved in glioma resistance to chemotherapeutic drugs, such as BCNU and TMZ 14, 15. It can repair DNA damage by removing alkyl groups from the O6 position of guanine 16. Both in vitro and in vivo data indicated tumor with low MGMT expression was more sensitive to TMZ chemotherapy 15, 17-22. Moreover, depletion of MGMT activity by O6-benzylguanine has been shown to reverse resistance 23-25.
In addition to MGMT-mediated DNA repair, both DNA damage-sensitive p53-conrolled cell cycle arrest and apoptosis pathways are likely to be involved in the tumor response to DNA-damaging treatments 26. Previous studies have shown p53 is one of the most frequently mutated genes in human glioma 27, 28. Here we want to see whether low MGMT expressing glioblastomas with different p53 expression level may have different clinical outcomes when treated with surgery, radiation and adjuvant temozolomide chemotherapy.
MATERIALS AND METHODS
Clinical material
Newly diagnosed glioblastomas with low MGMT expression (below 10% of positive cells in immunohistochemistry examination), who received tumor resection, conventional local irradiation and TMZ chemotherapy between Jan 2004 and December 2005, were included in the study. The histological diagnosis was made according to the World Health Organization (WHO) classification 29. Clinical data, including patients’ age at diagnosis, gender, preoperative Karnofsky Performance Status (KPS) score, tumor resection extent, MGMT expression level, p53 expression level, postoperative radiation, and chemotherapy were obtained from the medical charts. None of the patients had received preoperative radiation or chemotherapy. Tumor resection extent was evaluated according to enhancing MRI image no later than twenty-four hours after operation.. The study was approved by the ethics committee of Beijing Tiantan Hospital and a written informed consent was obtained from all patients.
Radiotherapy and chemotherapy
Postoperative limited-radiotherapy was delivered to the patient within 1 month after surgery. The total dose was 60 Gy, which was divided into 30 daily fractions of 2 Gy each. The initial treatment volume included the contrast-enhanced lesion and surrounding edema on the preoperative MRI scan plus a 2-3cm margin. After 46 Gy, the tumor volume included the contrast-enhanced lesion only. Provided that patient KPS score was over 70 and the tumor sample had a low level of MGMT expression, temozolomide were given 4 weeks after radiotherapy. The schedule was a daily dose of 150 to 200 mg per square meter of body-surface area for 5 days of every 28-day cycle. All patients were treated with at least two months of temozolomide. A total of six cycles were to be administered if no disease progression occurred and there were no irreversible hematological toxic effects.
Response evaluation
Treatment response was assessed by series of enhanced MRI scans. Tumor progression was defined according to the modified WHO criteria as an increase in tumor size by 25 percent or the appearance of new lesions 30. All timing was referenced to the date of operation, e.g. progression free survival (PFS) time as the interval between operation and radiographic progression, overall survival (OS) time as the period from operation to death due to any cause.
Evaluation of P53 expression level
Immunoperoxidase staining for p53 (Invitrogen) was performed on formalin-fixed, paraffin-embedded tissue sections following the standard protocol recommended by the manufacturer. Protein expression level was reestimated by a new-blinded observer. At least 1000 cells were counted in 10 different areas in each case using the 40×objective lens. Low expression was defined as below 10% of positive cells, staining restricted to the nucleus, in immunohistochemistry examination. The expression level of each patient was compared with the previous record. In case of a discrepancy, the observer reviewed the slides again to achieve a final result.
Statistics
The primary goals of the analysis were to uncover which parameters were associated with the clinical outcomes of the patients. The association between dichotomous variables was tested with the Chi-Square test or Fisher’s exact test. Survivor function curves were calculated with the Kaplan–Meier methods and difference was evaluated with the log-rank test. Multivariate cox models were used after univariate analysis. All reported P values are two-sided.
RESULTS
Totally, forty-six low MGMT expressing GBM patients were included in the study. All patients suffered tumor progression and 34 patients died after a median follow-up of 34 months (range: 24-57 months). The patients’ median PFS time was 315 days (95% CI: 276-354 days) and median OS time was 465 days (95% CI: 334-396 days).
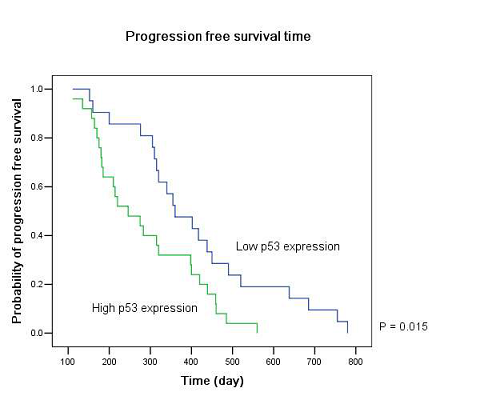
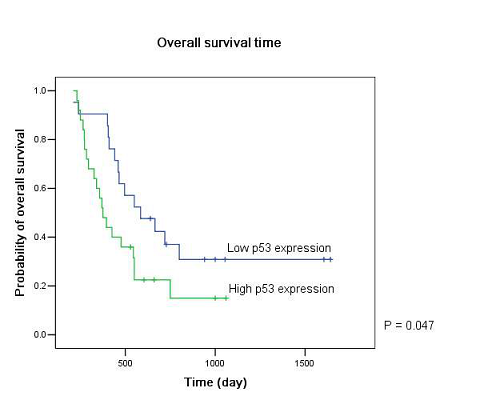
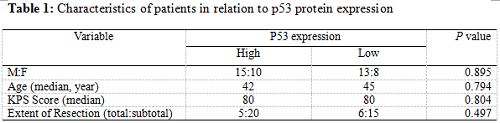
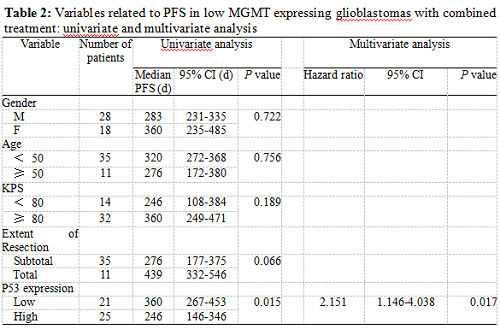
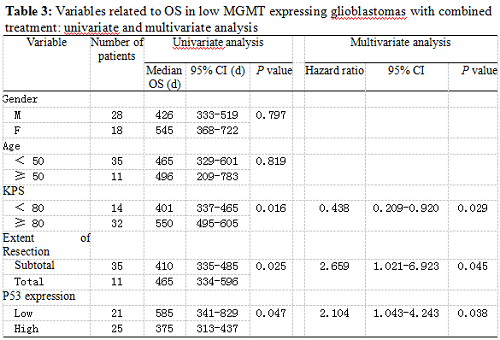
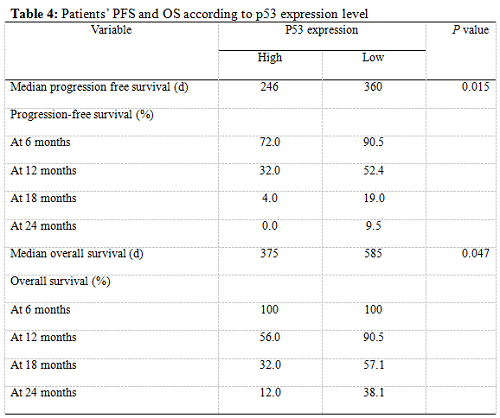
Twenty-five patients had high p53 expression and twenty-one patients had low p53 expression. No correlation existed between p53 expression level and patients’ gender (P =0.895), age (P = 0.794), KPS score (P = 0.804) or extent of tumor resection (P = 0.497) (Table 1). On both univariate and multivariate analysis, low p53 expression persisted as significant independent favorable prognostic factor for PFS (P = 0.017) (Table 2). In regards to OS time, preoperative KPS score (P = 0.016), tumor resection extent (P = 0.025) and p53 expression level (P = 0.047) were found to be statistically significant factors in the univariate analysis. They were all identified as significant independent prognostic factors for OS in the further multivariate analysis (P = 0.029, 0.045 and 0.038) (Table 3). The median PFS and OS time were 360 days and 585 days for low p53 expressing patients, which were 246 days and 375 days for those expressing high (Table 4 and Fig. 1). The differences were significant (P = 0.015, P = 0.047 respectively)
DISCUSSION
The role of TMZ is significant for glioblastoma 9, 31. TMZ spontaneously changes into its active metabolite 5-(3-methyl-triazeno)-imidazole-4-carboxamide, which can cause DNA damage by methylation of the O6 position of guanine, after oral administration. The fate of tumor cells may be then determined by activities of MGMT and some cell pathways responsive to cell damage, such as p53 and mismatch repair system. Researchers have proved MGMT can affect the clinical outcomes of TMZ in glioma 15, 17-20. But studies on the role of p53 to TMZ are not in accordance. Some studies in vitro reported that inactivation of p53 might sensitize glioma cells to BCNU and TMZ 32, 33. Other studies showed abrogation of wild-type function p53 strongly attenuated TMZ cytotoxicity 34. Bocangel et al proposed p53-mediated cell cycle arrest might be necessary for TMZ-induced cytotoxicity 35. Deferent cell lines used, other drug-resistant genes involved and different investigation methods might partially explain them. Clinical studies examining the relationship between treatment response and p53 status (including p53 protein expression and TP53 mutation) have largely failed to demonstrate a significant effect 36-38. Non-restrictive inclusion criteria and use of statistical methods, which were not sufficient to correct the possible bias, are important reasons. It appears that the prognostic information of p53 is complex and at best marginal, especially when compared to established parameters such as grading, age, etc 39. The prognostic value may require analysis of subgroups based on age and the status of specific markers such as MGMT, EGFR and bcl-2 40- 42.
Our results showed that the median PFS time was 315 days (95% CI: 276-354 days) and median OS time was 465 days (95% CI: 334-396 days) in these low MGMT expressing glioblastomas, which are in accordiance with the results by others 15, 43. Preoperative KPS score and tumor resection extent, which have been the most well-documented predictors of survival 36, 44, 45, were reaffirmed as significant independent prognostic factors for OS (P = 0.029 and P = 0.045). Age was not verified as a prognostic factor in the study for the small size of the patients. Low p53 expression was found to be a significant independent favorable prognostic factor for both PFS (P = 0.017) and OS (P = 0.038). The median PFS time and OS time were 360 days and 585 days for low p53 expressing patients, which were 246 days and 375 days for those expressing high (P = 0.015, P = 0.047 respectively). All these results indicated that low MGMT expressing glioblastomas with different p53 expression status had different clinical outcomes to the combined treatment. P53 played a role not only to the development of glial neoplasm but also to the response of radiation and adjuvant chemotherapy. High p53 expression may prompt the appearance of drug-resistant cell clones in these low MGMT expressing GBMs.
Several limitations are noted. First, p53 expression level was evaluated from paraffin-embedded tissue by immunohistochemistry method in the study. Western blot results using fresh specimen and study with sequencing data to identify the type of p53 are needed to further confirm it. Second, we only analyzed in the low MGMT expressing GBM patients. The contradiction of high price of the drug and low income of the patient makes it impossible for all patients to use the drug now in China. At the early stage, only patients with low MGMT expression, who are more sensitive to TMZ, are mobilized to use the drug in our center. Additional studies are required to explore the role of p53 in those high MGMT expressing GBM patients. Third, the results were drawn from a relative small population of Chinese. Studies with larger population and other ethnic groups are needed.
CONCLUSION
In this group of low MGMT expressing GBM patients treated with surgery, conventional local radiotherapy and adjuvant TMZ chemotherapy, low p53 expression was a significant independent favorable prognostic factor for both PFS and OS. In addition to MGMT, p53 may be another stratification variable in the future therapeutic trials.
REFERENCES
1. Avgeropoulos NG, Batchelor TT. New treatment strategies for malignant gliomas. Oncologist 1999; 4: 209-224
2. Laperriere N, Zuraw L, Cairncross G. Radiotherapy for newly diagnosed malignant glioma in adults: a systematic review. Radiother Oncol 2002; 64: 259-273
3. Grossman SA, O'Neill A, Grunnet M, et al. Phase III study comparing three cycles of infusional carmustine and cisplatin followed by radiation therapy with radiation therapy and concurrent carmustine in patients with newly diagnosed supratentorial glioblastoma multiforme: Eastern Cooperative Oncology Group Trial 2394. J Clin Oncol 2003; 21: 1485-1491
4. Medical Rsearch Council. Randomized trial of procarbazine, lomustine, and vincristine in the adjuvant treatment of high-grade astrocytoma: a Medical Research Council trial. J Clin Oncol 2001; 19: 509-518
5. Walker MD, Green SB, Byar DP, et al. Randomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant glioma after surgery. N Engl J Med 1980; 303: 1323-1329
6. Fine HA, Dear KB, Loeffler JS, et al. Meta-analysis of radiation therapy with and without adjuvant chemotherapy for malignant gliomas in adults. Cancer 1993; 71: 2585-2597
7. Stewart LA. Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet 2002; 359: 1011-1018
8. Wolff JE, Berrak S, Koontz Webb SE, et al. Nitrosourea efficacy in high-grade glioma: a survival gain analysis summarizing 504 cohorts with 24193 patients. J Neurooncol 2008; 88: 57-63
9. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005; 352: 987-996
10. Mirimanoff RO, Gorlia T, Mason W, et al. Radiotherapy and temozolomide for newly diagnosed glioblastoma: recursive partitioning analysis of the EORTC 26981/22981-NCIC CE3 phase III randomized trial. J Clin Oncol 2006; 24: 2563-2569
11. Cohen MH, Johnson JR, Pazdur R. Food and Drug Administration Drug approval summary: temozolomide plus radiation therapy for the treatment of newly diagnosed glioblastoma multiforme. Clin Cancer Res 2005; 11: 6767-6771
12. Henson JW. Treatment of glioblastoma multiforme: a new standard. Arch Neurol 2006; 63: 337-341
13. Stupp R, Hegi ME, van den Bent MJ, et al. Changing paradigms--an update on the multidisciplinary management of malignant glioma. Oncologist 2006; 11: 165-180
14. Hansen RJ, Nagasubramanian R, Delaney SM, et al. Role of O6-alkylguanine-DNA alkyltransferase in protecting against 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU)-induced long-term toxicities. J Pharmacol Exp Ther 2005; 315: 1247-1255
15. Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 2005; 352: 997-1003
16. Pegg AE. Properties of mammalian O6-alkylguanine-DNA transferases. Mutat Res 1990; 233: 165-175
17. Brandes AA, Tosoni A, Cavallo G, et al. Correlations Between O6-Methylguanine DNA Methyltransferase Promoter Methylation Status, 1p and 19q Deletions, and Response to Temozolomide in Anaplastic and Recurrent Oligodendroglioma: A Prospective GICNO Study. J Clin Oncol 2006; 24: 4746-4753
18. Chakravarti A, Erkkinen MG, Nestler U, et al. Temozolomide-mediated radiation enhancement in glioblastoma: a report on underlying mechanisms. Clin Cancer Res 2006; 12: 4738-4746
19. Fruehauf JP, Brem H, Brem S, et al. In vitro drug response and molecular markers associated with drug resistance in malignant gliomas. Clin Cancer Res 2006; 12: 4523-4532
20. Pollack IF, Hamilton RL, Sobol RW, et al. O6-methylguanine-DNA methyltransferase expression strongly correlates with outcome in childhood malignant gliomas: results from the CCG-945 Cohort. J Clin Oncol 2006; 24: 3431-3437
21. Gilbert MR, Armstrong TS. Management of patients with newly diagnosed malignant primary brain tumors with a focus on the evolving role of temozolomide. Ther Clin Risk Manag 2007; 3: 1027-1033
22. Nagane M, Kobayashi K, Ohnishi A, et al. Prognostic significance of O6-methylguanine-DNA methyltransferase protein expression in patients with recurrent glioblastoma treated with temozolomide. Jpn J Clin Oncol 2007; 37: 897-906
23. Ma J, Murphy M, O'Dwyer PJ, et al. Biochemical changes associated with a multidrug-resistant phenotype of a human glioma cell line with temozolomide-acquired resistance. Biochem Pharmacol 2002; 63: 1219-1228
24. Nutt CL, Noble M, Chambers AF, et al. Differential expression of drug resistance genes and chemosensitivity in glial cell lineages correlate with differential response of oligodendrogliomas and astrocytomas to chemotherapy. Cancer Res 2000; 60: 4812-4818
25. Quinn JA, Desjardins A, Weingart J, et al. Phase I trial of temozolomide plus O6-benzylguanine for patients with recurrent or progressive malignant glioma. J Clin Oncol 2005; 23: 7178-7187
26. Hickman MJ, Samson LD. Role of DNA mismatch repair and p53 in signaling induction of apoptosis by alkylating agents. Proc Natl Acad Sci U S A 1999; 96: 10764-10769
27. Stewart ZA, Pietenpol JA. p53 Signaling and cell cycle checkpoints. Chem Res Toxicol 2001; 14: 243-263
28. Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature 2000; 408: 307-310
29. Kleihues P, Cavenee WK. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Nervous System, Lyon, IARC Press, 2000.
30. Macdonald DR, Cascino TL, Schold SC Jr, et al. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 1990; 8: 1277-1280
31. Mrugala MM, Chamberlain MC. Mechanisms of Disease: temozolomide and glioblastoma-look to the future. Nat Clin Pract Oncol 2008
32. Xu GW, Mymryk JS, Cairncross JG. Pharmaceutical-mediated inactivation of p53 sensitizes U87MG glioma cells to BCNU and temozolomide. Int J Cancer 2005; 116: 187-192
33. Batista LF, Roos WP, Christmann M, et al. Differential sensitivity of malignant glioma cells to methylating and chloroethylating anticancer drugs: p53 determines the switch by regulating xpc, ddb2, and DNA double-strand breaks. Cancer Res 2007; 67: 11886-11895
34. Hermisson M, Klumpp A, Wick W, et al. O6-methylguanine DNA methyltransferase and p53 status predict temozolomide sensitivity in human malignant glioma cells. J Neurochem 2006; 96: 766-776
35. Bocangel DB, Finkelstein S, Schold SC, et al. Multifaceted resistance of gliomas to temozolomide. Clin Cancer Res 2002; 8: 2725-2734
36. Donato V, Papaleo A, Castrichino A, et al. Prognostic implication of clinical and pathologic features in patients with glioblastoma multiforme treated with concomitant radiation plus temozolomide. Tumori 2007; 93: 248-256
37. Ribeiro Mde C, Coutinho LM,Hilbig A. The role of apoptosis, cell proliferation index, bcl-2, and p53 in glioblastoma prognosis. Arq Neuropsiquiatr 2004; 62: 262-270
38. Shiraishi S, Tada K, Nakamura H, et al. Influence of p53 mutations on prognosis of patients with glioblastoma. Cancer 2002; 95: 249-257
39. Nieder C, Petersen S, Petersen C, et al. The challenge of p53 as prognostic and predictive factor in gliomas. Cancer Treat Rev 2000; 26: 67-73
40. Simmons ML, Lamborn KR, Takahashi M, et al. Analysis of complex relationships between age, p53, epidermal growth factor receptor, and survival in glioblastoma patients. Cancer Res 2001; 61: 1122-1128
41. Ganigi PM, Santosh V, Anandh B, et al. Expression of p53, EGFR, pRb and bcl-2 proteins in pediatric glioblastoma multiforme: a study of 54 patients. Pediatr Neurosurg 2005; 41: 292-299
42. Li SW, Jiang T, Li GL, et al. Impact of p53 status to response of temozolomide in low MGMT expression glioblastomas: preliminary results. Neurol Res 2008; 30: 567-570
43. Yaman E, Buyukberber S, Uner A, et al. Temozolomide in newly diagnosed malignant gliomas: administered concomitantly with radiotherapy, and thereafter as consolidation treatment. Onkologie 2008; 31: 309-313
44. Mineo JF, Bordron A, Baroncini M, et al. Prognosis factors of survival time in patients with glioblastoma multiforme: a multivariate analysis of 340 patients. Acta Neurochir (Wien) 2007; 149: 245-252
45. Gorlia T, van den Bent MJ, Hegi ME, et al. Nomograms for predicting survival of patients with newly diagnosed glioblastoma: prognostic factor analysis of EORTC and NCIC trial 26981-22981/CE.3. Lancet Oncol 2008; 9: 29-38
Figure legends
Figure 1: Kaplan–Meier estimates of (A) progression free survival and (B) overall survival according to p53 expression level in low MGMT expressing GBM patients by the log-rank test.




 京公網(wǎng)安備 11010802035500號(hào)
京公網(wǎng)安備 11010802035500號(hào)